Clinical
Module

Efficient & Timesaving
Optimized for Cost Reduction
Built for Patient Safety
Scalable & Flexible
Integration-Ready
GxP Compliant by Design
With TSS Clinical Module, you can monitor every kit in your trial with continuous temperature tracking from manufacturing to patient administration. The system intelligently evaluates temperature data against kit-level stability budgets. If an excursion occurs, the TSS Clinical Module automatically determines whether it falls within acceptable limits—powered by our advanced, validated calculation engine.
No manual review. No spreadsheets. Just fast, reliable decisions.
If a kit passes the stability check, automated approval is issued in seconds, with clinical site staff notified immediately. What once took days can now happen in moments—reducing quarantine time, increasing kit availability, and ensuring patient treatments stay on schedule.
Operational Benefits That Drive Trial Success and ROI

- Fewer kits in quarantine = lower storage and waste costs
- Less manual work = saved man-hours across sponsor and site teams
- Faster drug access = reduced risk of patient dropout
- Better data = stronger compliance and inspection readiness
Considering that a significant part of all clinical trial costs stem from patient recruitment, and most of the trial delays relate to patient availability, speeding up kit release has a measurable impact on trial performance and ROI. Each day a clinical trial is delayed can result in thousands of dollars in direct cost, and hundreds of thousands per day in lost potential revenue due to postponed market entry.
Safeguard Every Shipment. Justify Every Investment.
In clinical trials, the cost of temperature excursions goes far beyond product loss—it threatens timelines, patient safety, and the viability of trial outcomes. TSS Clinical empowers clinical operations teams to reduce risk dramatically by minimizing waste, avoiding costly resupplies, and protecting study timelines.
The result: greater confidence in your supply chain, stronger compliance, and a measurable impact on both cost and speed-to-market.
How TSS Clinical Module Automates Stability Monitoring
1. Temperature Data Collection
When preparing the shipment, USB or IoT-enabled temperature loggers are placed together with the products. Automated monitoring can also be applied during storage at clinical sites, as well as excursion based reporting when storage temperatures are exceeded. Because the module is logger-agnostic, you can use your preferred hardware, including mixed fleets across global trials.
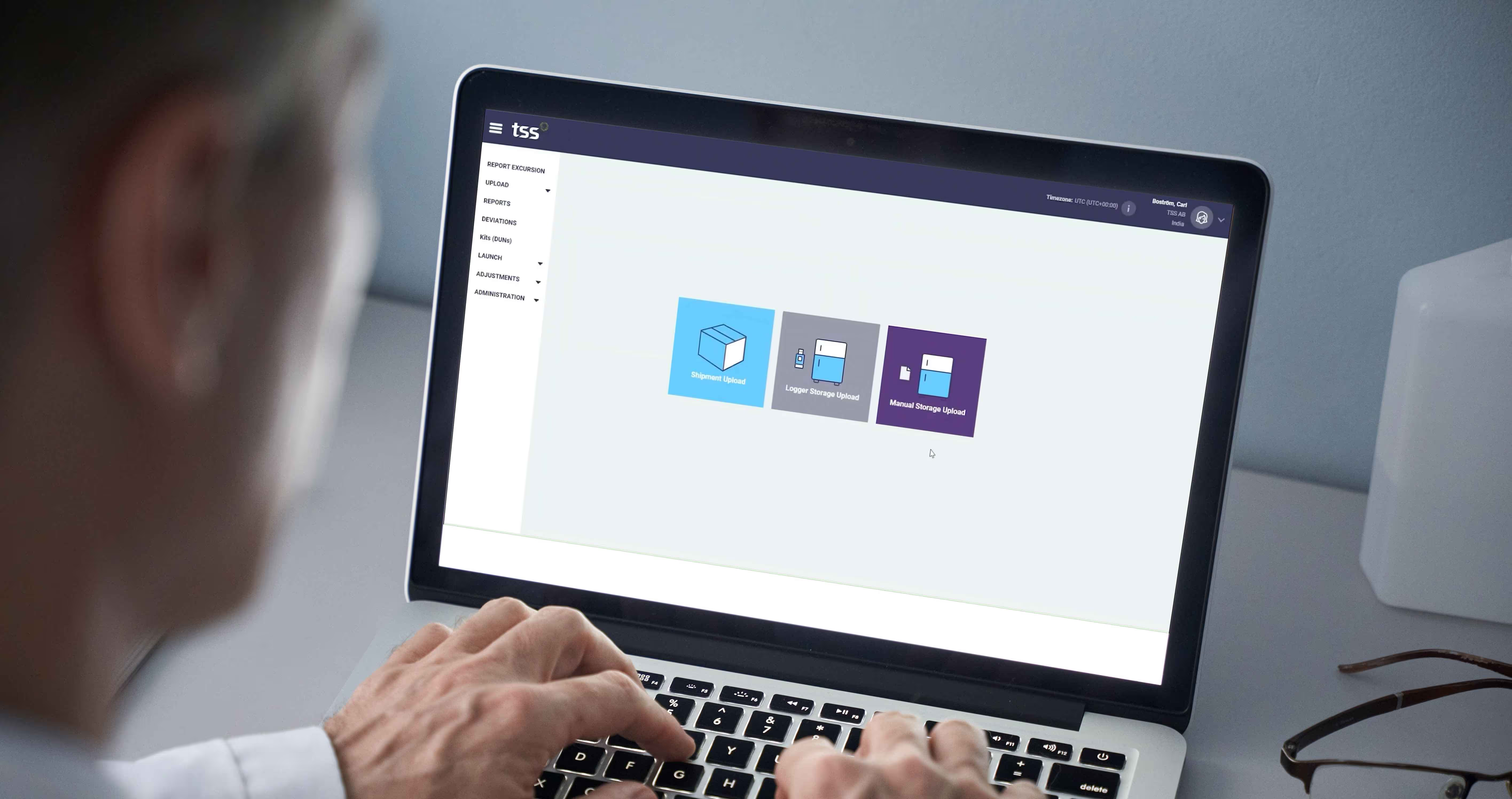
2. Automatic Upload to TSS Cloud Platform
All data is uploaded to the secure TSS cloud platform. This can be done manually or automatically through system integrations and gateways, depending on your choice of hardware. The TSS Clinical Module integrates easily with your existing clinical ecosystem, including:
- RTSM / IRT systems
- CTMS platforms
- Inventory and depot systems
Integrations are independent of choice of logger, whether TSS or 3rd party. Additional integrations can easily be made upon your request. We also have a standardized integration process that can be applied to most IRTs, accelerating the process with minimal input from the sponsor.

3. Excursion Detection and Stability Budget Check
Every time the data is uploaded, the system performs an automated stability evaluation. It compares the logged temperatures against the product-specific kit-level stability budget, determining whether an excursion occurred and if it’s within acceptable limits.
4. Integrated Notification and Release
The outcome is instantly communicated to the relevant systems and personnel. If the kit is cleared, it is automatically released, avoiding unnecessary delays. If a temperature excursion is detected, the platform uses its validated calculation engine to immediately determine the disposition. Most evaluations are completed in less than a second, providing an instant decision: OK to Use or Quarantine
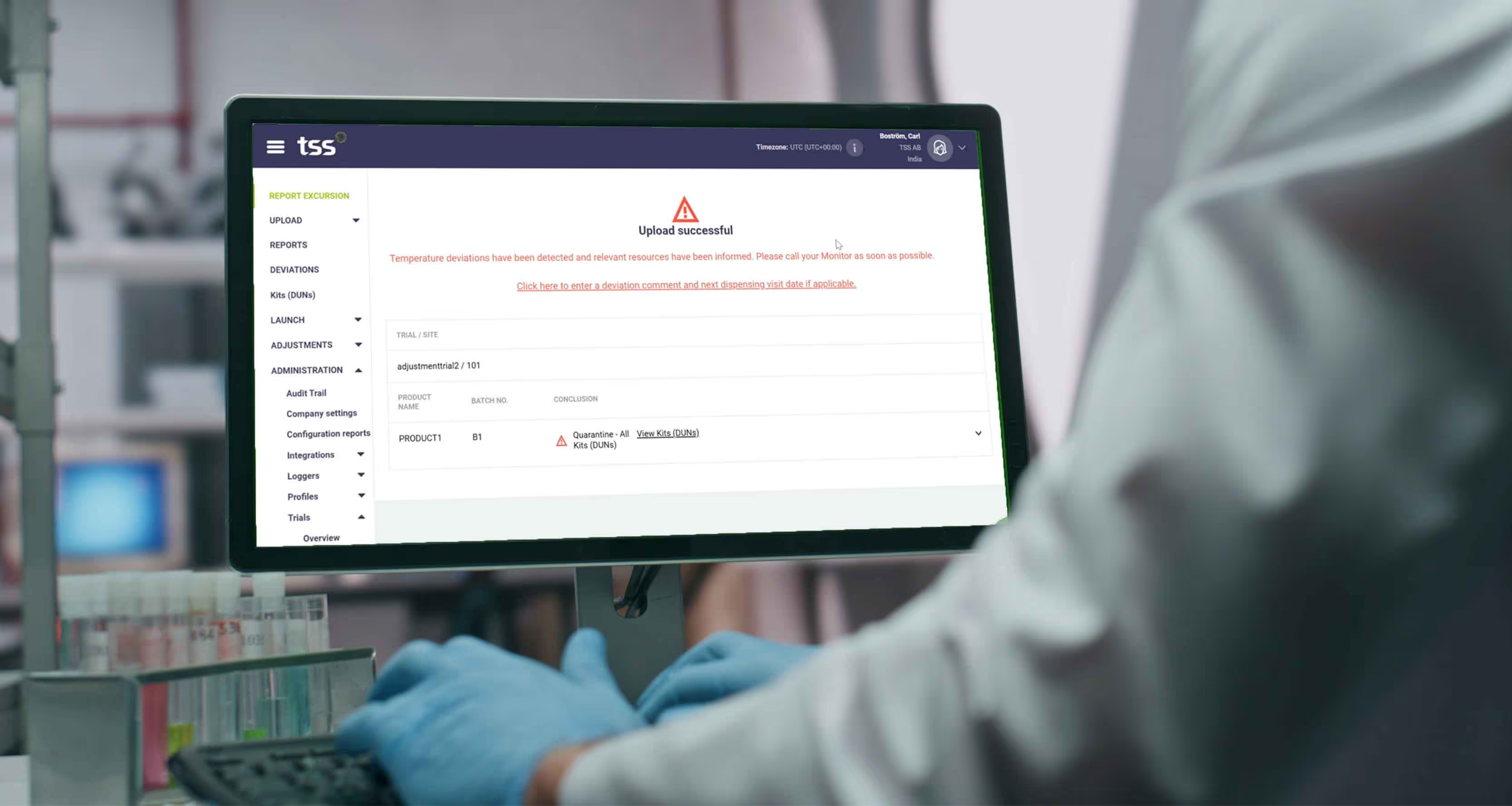
5. Full Lifecycle Tracking
Every temperature data point and disposition decision are tracked at the individual kit level, creating a complete audit trail from manufacturing to patient dispensing. This provides unparalleled traceability and inspection readiness.
6. Predictive Analytics and Continuous Optimization
All data is safely stored in the system, which offers dashboards and reports to identify trends, predict risk zones, and recommend improvements. This helps you proactively manage supply chain risks and improve planning for future trials.
Designed for Life-Science Excellence
Built for pharma, by pharma, the TSS Clinical Module meets the highest standards for compliance and automation. It is GxP-compliant and offers trusted, audit-ready performance to empower every stakeholder in the Clinical Trials process:
- Clinical Supply Chain Teams will gain full visibility of your IMPs in storage and transit. Reduce quarantine times, automate release decisions, and integrate effortlessly with your current systems!
- Quality Assurance & Compliance Professionals can meet inspection-readiness standards with automated, audit-traceable decision-making. Say goodbye to manual reviews and compliance gaps!
- Clinical Operations & Trial Managers will avoid costly trial delays. Deliver compliant medication to sites on time, with fewer protocol deviations and better site performance!
- CRAs & Site Staff may focus more on patients and less on paperwork. Receive real-time notifications on kit disposition and remove manual burden from site workflows!

Seamless Guidance & Data-Driven Optimization
With TSS, you’re never alone. Our Guidance and Support team make sure you get a smooth transition from implementation and onboarding to ongoing operational services. Whether you need training, troubleshooting, or compliance assistance, our experts are always ready to help.
Additionally, TSS Business Analytics Services turn your data into actionable insights, helping you identify inefficiencies, optimize supply chain performance, and improve compliance workflows.

Excursion Management for Clinical Supply
In collaboration with endpoint Clinical, we discuss current industry trends and the main challenges and opportunities that a unified approach incorporating IRT and end-to-end temperature solutions bring to sponsors.



