Clinical Trials
Supply Chain

Automated Temperature Excursion Analysis
End-to-End Compliance & Patient Safety
Seamless Integration & Scalability
We understand that in clinical trials, maintaining the integrity of investigational products is critical—not just for patient safety, but for the success of the study itself. Temperature excursions can compromise product stability, delay dispenses, and risk the validity of trial results. That’s why our cloud-based, automated monitoring solutions are designed to protect temperature-sensitive products throughout the supply chain—from depot to site. We help you safeguard product value, uphold protocol integrity, and deliver reliable outcomes for patients and sponsors alike.
Fit for Purpose: Temperature-Sensitive Clinical Trial Kits
Clinical studies vary in complexity and requirements—which is why we offer a range of temperature monitoring solutions to fit different study designs and operational setups. Whether you're running early-phase trials, global studies, or managing decentralized sites, we’ll help you identify the solution that best aligns with your needs, based on your protocols, infrastructure, and regulatory priorities.
End-to-end Clinical Trials Monitoring
TSS Clinical Module is an end-to-end solution, designed for the clinical trial supply chain. It aggregates data from pre-shipment, shipment, and storage on kit- or dispensing unit level (DUN) and compares it to stability data through an integration with the IRT/RTSM. This way drugs can automatically be released, or put in quarantine for further investigation. It is a cloud-based application that is technology agnostic, supporting a wide range of industry in-transit and storage loggers and integrations.
Reduces time for excursion management in clinical trials, from days to seconds
The benefits are many: less time spent on excursion handling for clinical sites and quality departments, minimized risk for manual errors and increased patient safety, timely completion of clinical trials and reduction of wasted products. The solution provide total peace of mind, ensuring safe storage and delivery to the patient.
Key Benefits:
- Full visibility from shipment to site to monitor temperature data across transit and storage, providing end-to-end stability tracking on a kit level.
- Automated excursion handling and release for comparing accumulated temperature exposure to stability budgets and enable instant, automated release decisions.
- Improved efficiency and patient access to reduces delays, manual workload, and the risk of patient dropouts by ensuring kits are released faster and reliably.
Ideal For:
- Mid to large-scale clinical trials where maintaining consistency, traceability, and speed across multiple sites and countries is critical.
- Temperature-sensitive investigational products requiring strict control and audit-ready documentation across both shipping and storage.
- Sponsors focused on reducing trial delays and improving patient dispense rates through faster product release and minimized operational bottlenecks.
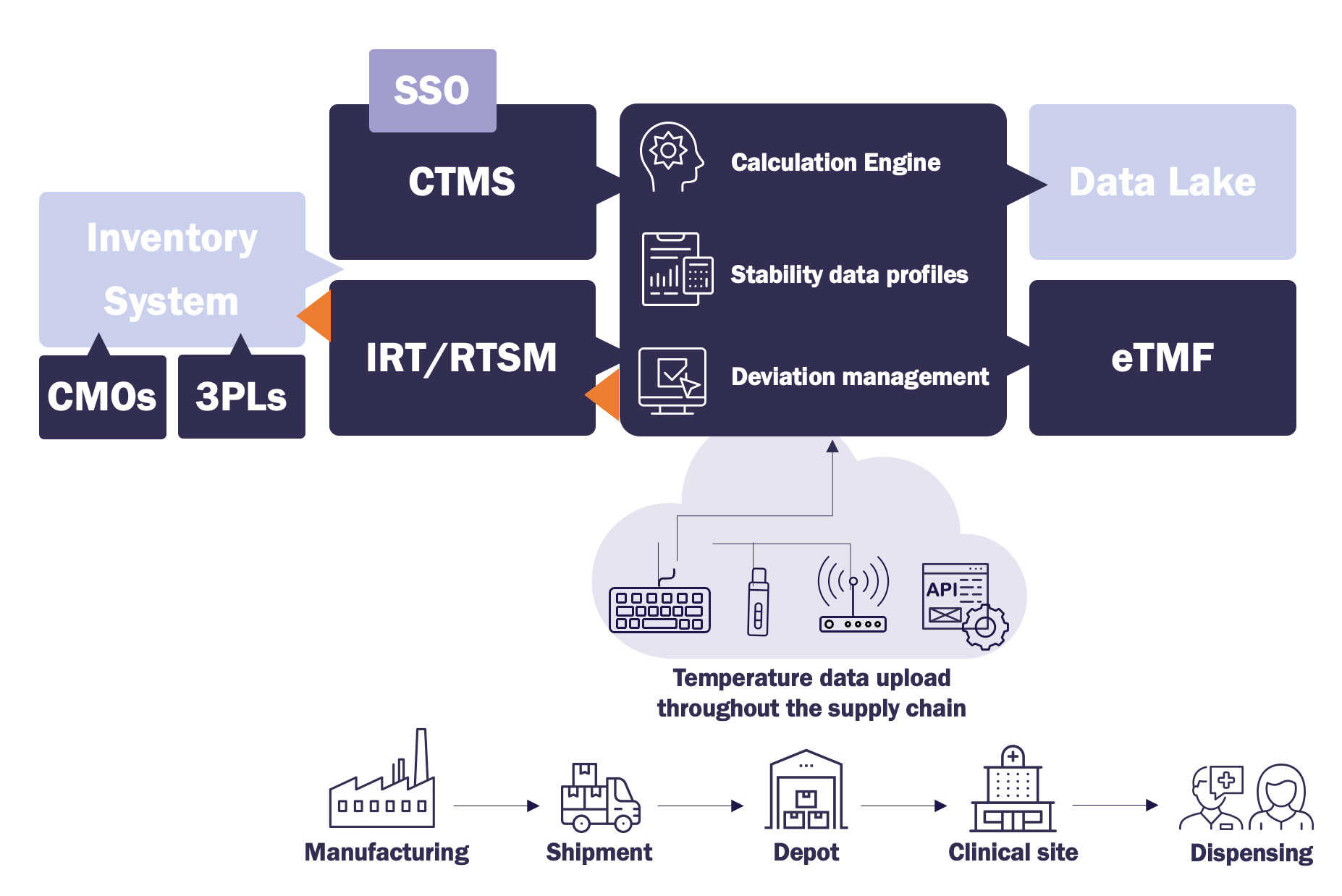
Manual Studies Plug-In for Clinical Trials Without RTSM
The Manual Studies plug-in in the TSS Clinical Module is designed to extend temperature monitoring and excursion management to early-phase or clinical trials that operate without an RTSM system. It provides a structured, compliant way to manage temperature-sensitive data, ensuring consistent oversight across all study types.
Key Benefits:
- RTSM-free temperature monitoring and excursion handling.
- Consistent processes across study types to standardize temperature workflows for early-phase and trials without randomization.
- Audit-ready and GxP-compliant to deliver validated data and full traceability to support regulatory requirements.
Ideal For:
- Early-phase clinical trials where full system integration is not yet in place but temperature oversight is still critical.
- Investigator-initiated and pilot studies that require compliant temperature monitoring without the complexity of an RTSM system.
- Sponsors seeking consistency across the portfolio by applying unified temperature monitoring standards regardless of study size or setup.

Seamless Integrations with Your Existing Systems
At TSS, we understand that a connected ecosystem is critical to modern pharmaceutical logistics. Our solution is designed to integrate effortlessly with your existing systems, including ERP, IRT, RTSM, Warehouse Management Systems (WMS), inventory management, and freight forwarder platforms.
This seamless connectivity ensures that data flows uninterrupted across your supply chain, automating workflows and providing a unified view of operations. By synchronizing the solutions at TSS with your current technological infrastructure, you can minimize disruptions and maximize efficiency. With scalable and secure integrations you can focus on delivering safe, high-quality trial products without the hassle of manual data transfers.
Learn more about integrations >
Built for Compliance in Regulated Supply Chains
The solutions at TSS are designed to meet the rigorous regulatory demands of the global clinical trial supply chain, supporting FDA and EMA regulations. As an ISO 9001 certified organization, we follow robust quality management principles that ensure consistency, reliability, and continuous improvement across our systems and processes. By automating data management and minimizing manual effort, we help your organization remain fully compliant and inspection-ready at all times.

Trusted Guidance, Support & Business Analytics
- Benchmark: Identify your current performance relative to industry leaders.
- Evaluate: Assess gaps in automation, integration, and analytics.
- Implement: Adopt automatic monitoring and predictive analytics.
- Optimize: Use insights to drive continuous improvement in waste, inventory, and compliance.
With TSS, you’re never alone. Our Guidance and Support team is available at every stage—from implementation and onboarding to ongoing operational services—ensuring that your team is fully equipped to maximize the value of your monitoring solution. Whether you need training, troubleshooting, or compliance assistance, our experts are always ready to help.
Read more about support >
Beyond monitoring, TSS Business Analytics Services turn raw data into actionable insights, giving you greater visibility into inefficiencies, temperature excursions, and supply chain performance. By leveraging powerful analytics, you can optimize routes, reduce waste, and make data-driven decisions to improve overall logistics efficiency.
View analytics services >
Which Solution is Right for You?
We offer scalable, compliant, and user-friendly temperature monitoring solutions fit for purpose. No matter your study supply chain complexity, risk level, or automation needs, we have an easy-to-implement solution for you.
Insights
Let us explain further

How do I get started?

Getting started with digitalization of temperature control with TSS solutions is often faster than expected, and we will guide your through the process of setup, configuration and process development. Read more about how it works here

What performance can you guarantee?

24/7 monitoring, backup and redundancy for 100% peace of mind.

Does TSS offer real-time monitoring solutions?

Yes, we offer both real-time monitoring for shipment and storage, including software, data loggers and 24/7 Control Tower services including device management and intervention.

Can TSS integrate with other systems?

Yes, one of our specialist areas is integrations with 3rd partner software for operational efficiencies and cost savings. We have experience of integrating several ERP, IRT, Freight Forwarders, WHM-systems and more. Read more about our integrations here










_B.avif)
























.avif)























.avif)