Sustainable Temperature Management: A Key to Greener Clinical Trials
One area where big improvements can be made is in the management of temperature-sensitive products, like vaccines, biologics, and experimental drugs, in clinical trials. These products need careful temperature control to keep them safe and effective. But as sustainability becomes a bigger priority, it's time to rethink how we manage temperature—without affecting product quality, patient safety, or trial outcomes.
The Environmental Impact of Clinical Trials
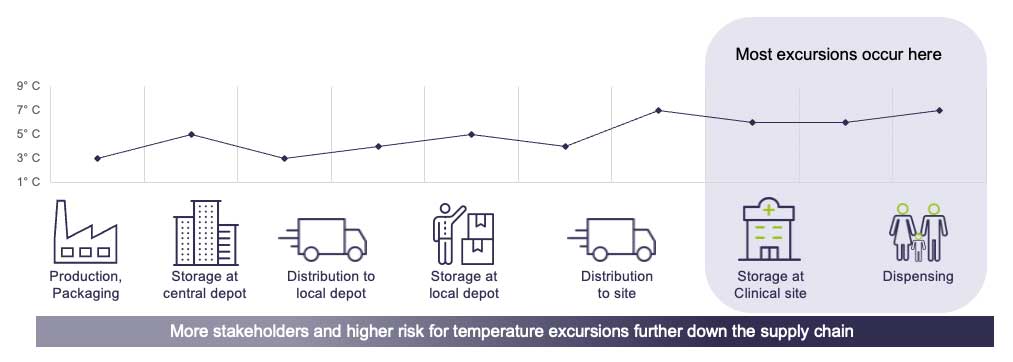
Clinical trials use a lot of resources. In addition to the costs of staff, facilities, and patient recruitment, the transportation and storage of temperature-sensitive products have a big environmental impact. The cold chain—how temperature-sensitive products are transported and stored—often requires a lot of energy, frequent shipping, and heavy packaging, all of which contribute to carbon emissions and waste.

The pressure to reduce this impact is growing. Regulatory bodies are beginning to require more sustainable practices, and customers expect phama companies to take responsibility for their environmental impact. The pharmaceutical industry has already made progress with green manufacturing, sustainable packaging, and more efficient systems. But the supply chain can become more sustainable too.
Energy-Efficient Temperature Control
Cold chain logistics, which refers to the transportation and storage of temperature-sensitive drugs, is key to clinical trials. But older, traditional refrigeration systems are energy-intensive and often use harmful refrigerants. The good news is that there are now energy-efficient alternatives that can maintain the right temperature while reducing the environmental impact. For example, new refrigeration systems use natural refrigerants and low-energy cooling, reducing the carbon footprint. These systems still provide the best conditions for clinical trial products but do so with less energy and less environmental damage.

In addition, digital tools and analytics can be used to optimize the supply chain. By planning transportation routes and monitoring temperature in real-time, companies can use less fuel and reduce emissions. These smarter logistics help save energy while ensuring that clinical trial materials are always kept at the correct temperature.
IoT and Automation
Temperature monitoring is crucial in clinical trials, especially as regulations around product safety become stricter. With IoT-based temperature loggers, conditions of the products can be monitored automatically, ensuring they stay within the correct temperature range throughout the trial process. The temperature data points are analyzed using cloud-based software, integrated with the technological infrastructure of the clinical trial supply chain, ultimately providing a seamless end-to-end perspective of the process.
These technologies also help reduce waste. By using automated tools for cumulative excursion calculation, the release of products can be made in seconds rather than days, which reduce inventory holding costs and the need for resupply. While releasing more drugs to patients faster, these automated applications also prevents the need for replacement shipments and their respective carbon footprint. With a system that ensures less products end up in quarantine, the industry can ultimately reduce overage in production, which has a substantial environmental impact.
Another benefit of these smart systems is that they reduce the use of paper-based processes, further decreasing waste. Data can be stored and analyzed digitally, making it easier to track and monitor temperature without using printed reports and data sheets.
Sustainable Packaging
Packaging plays an important role in temperature management. Temperature-sensitive products need to be packed with insulation and cushioning materials to keep them safe during transport and storage. But traditional packaging materials often rely on plastics and other non-recyclable materials.
To meet sustainability goals, the variety of eco-friendly packaging alternatives are increasing on the market. For example, reusable or biodegradable insulation and recyclable materials are becoming more common. These materials still protect the products but create less waste. This shift helps companies reduce their environmental impact while also meeting the growing demand from patients and stakeholders for more sustainable practices.
Designing Sustainable Clinical Trial Processes
As clinical trial designs continue to evolve, it’s important for professionals in the industry to include sustainability at every stage of the trial—from the planning phase to execution. By adopting energy-efficient temperature control, using smart temperature monitoring tools, and focusing on sustainable packaging, the pharmaceutical industry can cut down its environmental footprint while still ensuring patient safety and product quality.
The pressure to be more sustainable is not just coming from regulations; it’s also driven by the expectations of patients, investors, and other stakeholders. More and more, these groups want the healthcare industry to take responsibility for its impact on the planet.
It's time to embrace sustainability in the supply chain when designing the next generation of clinical trials. By doing so, we can create greener trials, lower costs, and contribute to a healthier future for both patients and the planet.
Whether you run a few clinical trials per year, or several large-scale studies, there is a TSS Solution to support you with efficient temperature monitoring.Temperature Monitoring in Clinical Trials Supply Chain > |
Sources
FDA – Sustainable Design and Manufacturing
https://www.fda.gov/industry/environmental-impact
World Health Organization (WHO) – Sustainability in Pharmaceutical Supply Chains
https://www.who.int/health-topics/sustainable-development-goals
Gartner – The Future of Supply Chain in Healthcare
https://www.gartner.com/en/insights/supply-chain
Pharmaceutical Technology – Cold Chain Sustainability
https://www.pharmtech.com/view/sustainability-advances-cold-chain
McKinsey & Company – Sustainable Manufacturing Practices in Pharma
https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights
The Green Chemistry Institute – Green Pharmaceutical Manufacturing
https://www.acs.org/content/acs/en/greenchemistry.html
Environmental Protection Agency (EPA) – Green Refrigeration Technologies
https://www.epa.gov/ozone-layer-protection/green-refrigeration
IoT in Pharmaceutical Supply Chain – A Green Revolution
https://www.pharmaceuticalcommerce.com/
You may also be interested in

TSS Keeps EcoVadis Silver Medal Despite Tougher Standards in 2024

TSS Celebrates WTISD 2024: Embracing Innovation, Digitalisation, and Sustainability

Join us at GCSC US 2022
You may also be interested in

TSS Sponsors the 27th Annual Clinical Trial Supply Europe 2026 in Barcelona


