3 steps to accelerate Decentralized trials & Release to patients

One thing that the pandemic taught us was that we can do things much faster than we initially thought. However, when it comes to waiting for a conclusion on the temperature excursion, it's still normal that it takes days, or even weeks. Recently, a leading pharmaceutical company decided to go from manual Excel files and move to an automated calculation engine, to release products in seconds instead of days.
Other sponsors have implemented automated technology at clinical sites to help site staff spend less time on manual tasks and more time with patients. Digitalizing and automating temperature management also enables a seamless and fast release to patients in decentralized trials.
1. How to achieve a 93% time-reduction in temperature management
When looking at the temperature chain for trial products, every time a temperature excursion occurs, the evaluation of that excursion takes a lot of time and manual effort. In the end, most of those excursions do not result in a deviation and the product is in fact OK to use. Potential rescheduling of patient visits could have been avoided.

Apart from the fact that time savings are cost savings, why do we want to reduce the time the product spends in quarantine?First, to reduce the failed patient visits. From the patient's perspective, this might be a lifesaving treatment. But also, if they are driving several hours to get onto site to get this dispensed, they are not very happy to be rescheduled and must do that same journey again.
When reducing the waiting time and the rescheduling, we may as well lower the risk of patient dropouts, which of course is very expensive and can even compromise the total length of the trial.Timeliness of the trial also gets increasingly important with higher amounts of personalized medicine and specialized trials.

Having an automated way of managing temperature excursions can also contribute to sponsor attractiveness, as we are offering the site one single source of truth, instead of physical binders of paper files. The calculation engine provides inspection readiness as all data is at hand, just one click away. All data is fed to the eTMF system and easily exported if needed.
Reducing the waiting time for a conclusion on a temperature excursion, can help prevent protocol deviations due to patients being dispensed with a temperature affected product during that time.
By reducing the time that kits are in quarantine due to evaluation of temperature excursions, there is less need for sending replacement products to sites. Reducing the automatic fill may even reduce the overproduction of clinical trial products, which has substantial effects on costs and sustainability.
Assuring the quality of the study drugs across the entire clinical supply chain through to Patients.
Implementing an automated calculation engines can reduce the time to evaluate a temperature excursion from days down to one second. For most excursions that, based on stability data, does not result in a deviation, the site knows instantly that the product is OK to use.
For the part of excursions that need further evaluation, the site is notified to keep products in quarantine and await a conclusion from the Sponsor. The Sponsor is also directly notified to take action on a potential deviation and can process the conclusion within the calculation engine.
The calculation engine integrates key components in the clinical ecosystem. It holds the stability data profiles of the product and compares every new temperature upload from shipments and storage with the stability data set on kit level. From the CTMS system integration comes trial and site data, and from the IRT orRTSM, shipment ID's and Kit IDs and their statuses are received. The calculation engine can also integrate with sponsor data lakes or eTMF systems.
Over the recent couple of years, some sponsors have taken automation even further, by implementing wireless technology at clinical sites. This technology is increasingly popular, not only with the sponsors, but also from the clinical site perspective.
2. How to boost site efficiency in temperature monitoring by 90%
Clinical sites are burdened with complexity as different trials and sponsors operate various hardware and software solutions. Also, there is a mix of high- and low-quality devices. Many temperature monitoring devices provide low quality data, risk of human error, as well as a high failure rate.
When a wireless solution for clinical site temperature monitoring was offered, there was instantly a pull from the sites to get their hands on this technology. They realized it would save them a lot of time, but as well, provide the future benefit of potentially being able to have just one set of hardware, feeding the data to different sponsors. Equally important, it is proven to be plug and play solution, with close to zero support tickets on how to setup the equipment.
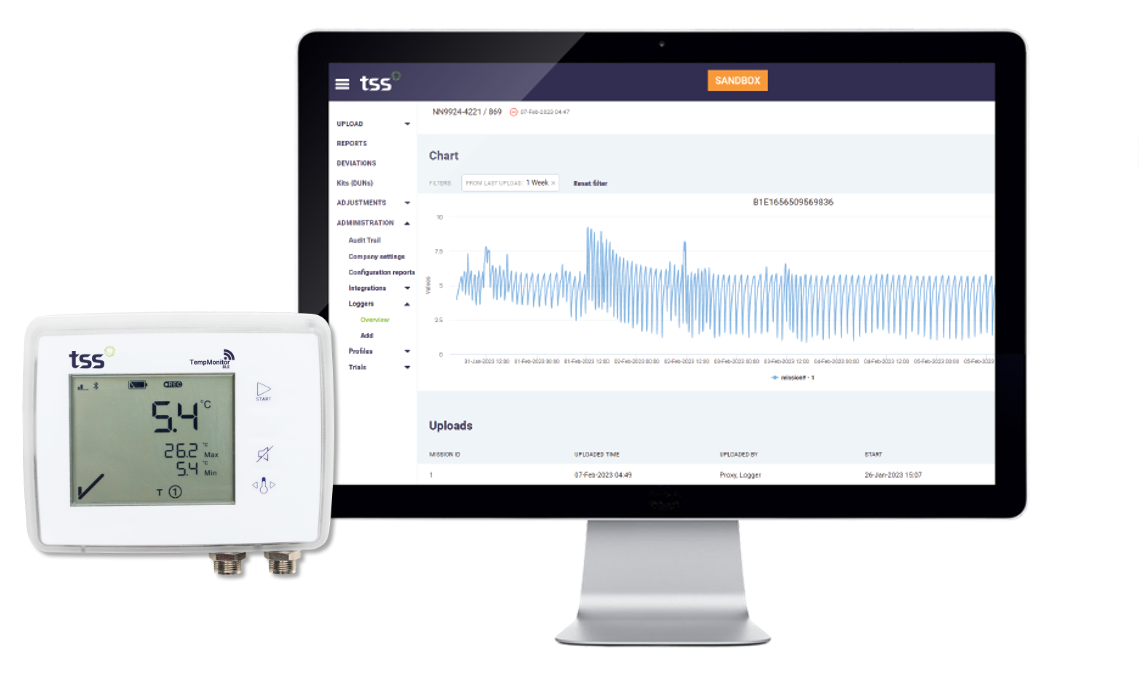
Temperature data is sampled every 5 minutes and sent automatically over cellular networks or Wi-Fi to the online calculation engine. It provides instant notifications on temperature alarms so that site staff can act faster to prevent temperature excursions in their storage areas. It also provides a global real-time overview for the sponsor, as all data is available in the calculation engine.
The benefits of site efficiency by fully connected monitoring
- A reduction of 90% in time spent on temperature monitoring activities for clinical sites.
- A reduction in temperature monitoring activities for the Sponsor team by 1700 hours per year.
- Ability to prevent future excursions and reduce wasted trial products.
With the increase of more sensitive medicines, the ability to prevent future temperature excursions becomes increasingly important. From the big data set of temperature measurements from these devices, predictive analytics or machine learning, allows for creating alerts before product quality is compromised. For example, in failing storage environments, we can predict future extreme temperatures, and save more products from being wasted. This type of data trending is already used in detecting risk levels in shipment routes.
3. How to enhance patient experience with a unified digital release
The benefits of digitalizing temperature management, and automating excursion reporting, create a foundation for successful decentralized trials. The TSS calculation engine, expands to cater for releasing at patient's homes. As a first step, the distributor can upload USB shipment temperature loggers to the calculation engine. In the near future will the upload be made seamlessly by a Bluetooth logger, integrated with the patient app and the IRT, for a unified digital release at patient's home.
You may also be interested in
Join Us at LogiPharma Europe 2025

TSS launches innovative Bluetooth powered temperature monitoring solutions

Turn days into seconds at clinical sites worldwide
You may also be interested in

Can You Rely on the Results from Your Clinical Trials?


